Wetting of solid surfaces and powders
- The forces acting on a drop on the solidsurface (Figure 1.4a) are represented by
Young’s equation:
γS/A = γS/L + γL/A cos θ
where γS/A is the surface tension of the
solid,
γS/L is the solid–liquid interfacial tension,
γL/A is the
surface tension of the liquid and θ is the contact angle.
- The tendency for wetting is expressed by the spreading
coefficient, S, as:
S = γL/A (cos θ – 1)
- For complete spreading of the liquid over the solid surface,S should have a zero or positive valve
- If the contact angle is larger than 0°, the term (cos θ – 1) will be-ive, as will the value of S.
- The condition for complete, spontaneous wetting is thus a zero valve the contact angle.
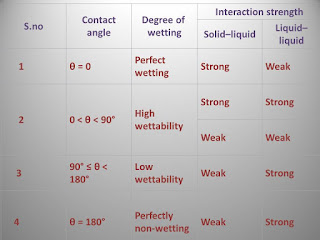
- The effectiveness of immersional wetting may be related to the contact angle which the solid makes with the liquid–air interface.
- Contact angles of greater than 90° indicate wetting problems,for example when the drugs are formulated as suspensions.
- Examples of very hydrophobic (non-wetting) drugs include magnesium and aluminium stearates, salicylic acid,phenylbutazone and chloramphenicol palmitate.
- The normal method of improving wettability is by the inclusion of surfactants in the formulation. The surfactants not only reduce γL/A but also adsorb on to the surface of the powder, thus reducing γS/L. Both of these effects reduce the contact angle and improve the dispersibility of the powder.







0 comments:
Post a Comment